Country: Mozambique
Administrative region: Maputo (Province)
Central co-ordinates: 26.0286 S, 32.92730 E
Area: 132km²
Qualifying IPA Criteria
A(i)Site contains one or more globally threatened species, B(ii)Site contains an exceptional number of species of high conservation importance
IPA assessment rationale
KaNyaka Island qualifies as an IPA under criterion A. With its recognised botanical importance at both national and international levels, the island is home to eight threatened taxa that trigger criterion A(i), including two Endangered species, Ecbolium hastatum and Solanum litoraneum, and six Vulnerable taxa, Adenopodia schlechteri, Dioscorea sylvatica, Psychotria amboniana subsp. mosambicensis, Tephrosia forbesii subsp. forbesii, Tephrosia forbesii subsp. inhacensis, Zostera capensis. Although not IPA trigger, it is important to highlight the presence of a near-endemic cycad, Encephalartos ferox subsp. ferox, assessed as Near Threatened at species level. Overall, there are 12 endemic species within this IPA, falling within the top 15 sites for Mozambique’s endemic and range restricted species and therefore triggering sub-criterion B(ii) for this IPA.
Site description
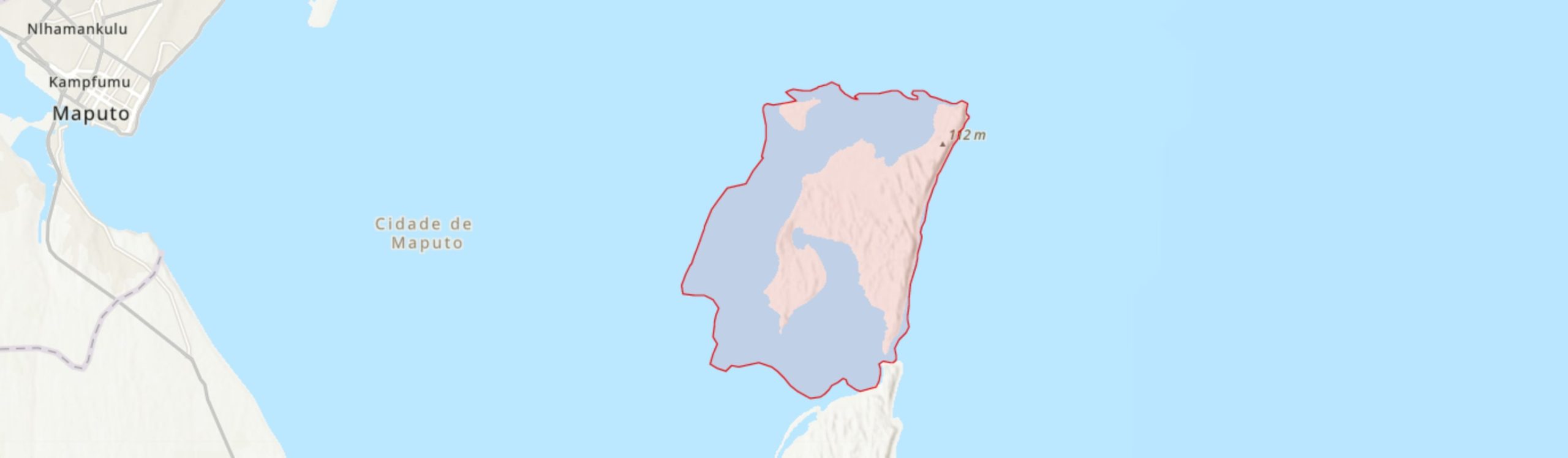
KaNyaka (also known as Inhaca Island), is situated in Maputo Bay (-26.02°, 32.94°) off the coast in south-east Mozambique. It forms a barrier separating Maputo Bay from the Indian Ocean (Mogg 1967). To the south is the Machangulo Peninsula separated from the Island by an inlet about 800 m wide and up to 15 m deep (Hobday 1977). To the north-west, is Portuguese Island of about 3.7 km2 which is included within the boundary of this IPA. The intertidal zone of KaNyaka within Maputo Bay is also included within the boundary of this IPA as it is home to important seagrass communities. In administrative terms, KaNyaka Island, along with Portuguese Island, are under the Maputo municipality and is recognised as a separate municipal district.
KaNyaka Island has a high-energy wind- and wave-dominated oceanward shore with a steep sandy beach and high vegetated coastal dunes. In terms of elevation, the highest point, Mount Inhaca, is over 100 m high (Hobday 1977). The total area of the Island, which resembles a distorted “N” (Muacanhia 2004), is just over 40 km2, with a maximum length of about 12 km from Ponta Mazondue to Ponta Torres in the south. The total area of the IPA, which also includes the Portuguese Island and the foreshore of the KaNyaka Island, is about 132 km2. It is home to 6100 inhabitants distributed in three main areas including Nhakene (over 1500 residents), Ribjêne (2100) and Inguane (2500) (Sörbom & Gasim 2018). Alongside the local inhabitants, the island and the adjacent Machangulo Peninsula is a prime tourist destination in southern Mozambique, a particular highlight for cruise liner tourism, hence various tourist facilities have been established, particularly along the coastline.
During the colonial rule, the Islands (KaNyaka and Dos Portugueses) and Maputo Bay had a European presence, especially British and Dutch-Portuguese and, as a result, Ilha dos Portugueses is known by several different names (Adam et al. 2014). KaNyaka Island was occupied by the British for over half a century, from 1823 to 1875 (Portugal & Matos 2018). The Island was used to patrol and control activities related to the trade in ivory and enslaved people in the region.
Botanical significance
There are 455 species recorded from KaNyaka (Matimele & Timberlake 2020). Darbyshire et al. (2019) places KaNyaka among sites with more than 20 endemic or range restricted taxa in Mozambique, therefore recognising it as one of the most important sites nationally for botanical richness.
KaNyaka Island falls entirely within the Maputaland Centre of Endemism (CoE) and several Maputaland endemic or near-endemic plant species have been recorded, a particularly significant species is Helichrysum moggii (LC), only known from KaNyaka Island and Santa Maria Cape of the Machangulo Peninsula. Other Maputaland endemic or near-endemic species include Coastal Jackal-berry (Diospyros inhacaensis), Coastal Bitter-tea (Distephanus inhacensis), Tritonia (Tritonia moggii), and Dune Knobwood (Zanthoxylum delagoense). Although T. moggii has been recorded as far northwards as Inhambane, the species is highly concentrated on the KaNyaka Island. Zanthoxylum delagoense, a Maputaland near-endemic and a Mozambican endemic (Matimele 2016), is also present within the open forests of this IPA.
There are eight globally threatened species present on KaNyaka Island. One of these species, Ecbolium hastatum (EN), is only known from about five localities, all restricted to southern Mozambique, including Ponta Ponduine on this island. A second Endangered species, Solanum litoraneum, is also endemic to coastal southern Mozambique and is threatened by the development of its coastal dune habitat (Knapp, in press), although the population within this IPA is relatively secure and therefore may represent an important opportunity to conserve S. litoraneum.
Alongside these Endangered species are six Vulnerable taxa, including two subspecies of Tephrosia forbesii, subsp. forbesii and subsp. inhacensis. The latter taxon, Tephrosia forbesii subsp. inhacensis, is known only from the western dunes of KaNyaka Island and is therefore endemic to this IPA. While much of this subspecies’ habitat appears intact, encroaching agriculture and housing (both residential and tourist) threaten this taxon with extinction (Langa et al. 2019). Two other Vulnerable taxa, Psychotria amboniana subsp. mosambicensis and Adenopodia schlechteri, also have restricted ranges, with both species’ endemic to southern coastal Mozambique (Burrows 2018).
Dioscorea sylvatica (VU), contrastingly, has a wide distribution, occurring from South Africa to Zambia, and has been collected in woodland and around abandoned machambas within this IPA. Despite its broad distribution, this species has a history of overharvesting. Tubers of this species have medicinal properties and, in the 1950s, they were harvested on an industrial scale to manufacture cortisone and other steroid hormones (Williams et al. 2008). Today this species is still harvested and sold locally, particularly in South Africa, although there is no record of this species being harvested at this site.
While the coastal dune communities of KaNyaka are a major botanical importance, hosting most of this site’s threatened and endemic species, the seagrass communities in the intertidal zone are also significant. The Vulnerable species, Zostera capensis, occurs in both the south (Saco and Banco) and north bays of KaNyaka as well as in the northern intertidal areas of Maputo city, in areas with fine, muddy sediments exposed at low tide. Although found across coastal areas of southern and eastern Africa, this species colonises areas slowly and is sensitive to pollution and sedimentation, while in Mozambique there is a specific threat of disturbance associated with shellfish harvesting (Short 2010; Bandeira and Gell 2003). The Z. capensis meadow in the southern bay of KaNyaka is the largest in the world, and falls within Ponta do Ouro Marine Partial Reserve, and so this IPA offers a great opportunity to conserve this species (Bandeira et al. 2014a).
Elsewhere in the intertidal zone, on the west coast of the island, is a population of the near-endemic seagrass Thalassodendron leptocaule. This species has been assessed as Near Threatened and, although threatened elsewhere by tourism and other coastal activities, the KyNaka populations are relatively secure as all the waters off this island fall within Ponta do Ouro Marine Partial Reserve (Duarte et al. 2014). Nevertheless, this species occurs at only one site at Inhaca, at the northernmost point over looked by the lighthouse (Farol de Inhaca). Both Z. capensis and T. leptocaule are of great ecological importance, providing habitat, shelter, nurseries and foraging grounds for marine invertebrates and fish (Adams 2016; Browne et al. 2013). In total, nine different species of seagrass have been recorded around KaNyaka, representing over three-quarters of Mozambican and over 16% of global seagrass species (Bandeira 2002).
There are a number of useful species present within this IPA. Although none of these species are of conservation concern or restricted to KaNyaka, they are highly important for the local communities. The mangroves, found on the Maputo Bay coastlines of KaNyaka, consist of species common throughout these habitats in Mozambique but are locally important as a source of timber and fuelwood while also providing coastal stabilisation by preventing erosion, regulating sedimentation and protecting against tidal surges (Paula et al. 2014). In addition, a number of species are voluntarily protected on the island, including Strychnos spinosa, Syzygium cordatum, Sclerocarya birrea subsp. caffra (Marrula), as fruits of these trees are consumed locally. Of these species, particular emphasis is given to the Marrula tree, which has a long history of being encouraged by local people. The fruits of this species are used to make a traditional beverage associated with celebrations in the community, as such it is regarded one of the most important indigenous trees (Mogg 1967).
Although the island has been well studied over the years, botanical surveys still yield new records for KaNyaka, for instance, the widespread African tree species Cassipourea malosana, was only recently recorded on the island (Massingue 2019). This suggests that further new records, potentially including further species of conservation concern, may be uncovered in the future.
Habitat and geology
The emergence of KaNyaka is linked to the formation of Maputo Bay that resulted from a recent Holocene transgression (Achimo et al 2014). This island consists of a calcareous sandstone base which has been overlain with notably high dune ridges (Muacanhia 2004). The geomorphological dynamics of the coastal dune systems of KaNyaka together with a prevailing sand deposition, and erosion dynamics, in shallow waters continues to shape these islands and the entire Maputo Bay.
The landscape of the island is made up of two long ridges (Mount Inhaca in the north-east and the Barreira Vermelha in the west) trending north-south with an undulating plain between them presenting smaller ridges separated by low sandflats or swampy terrain (Macnae & Kalk 1967; Hobday 1977). The smaller ridges can reach 40 m high, and they are about 5 to 6 km apart west to east. The soils are mainly sandy, which vary from brown in non-disturbed forest patches, to light yellowish brown in other vegetation types at different phases of development (Campbell et al. 1988).
The climate of KaNyaka Island is tropical (Macnae & Kalk 1962) with two main seasons over the year, including a rainy and warm season from October to March, followed by a dry and cooler season ranging from April to September (Muacanhia 2004). The island is usually humid but has a surprisingly low rainfall of around 600 mm per annum. The mean annual temperature is 22 to 23°C, although temperature varies considerably throughout the year, with a maximum of 37°C and a minimum around 12°C (Muacanhia 2004).
Dune vegetation is most dominant on the east coast of KaNyaka, although is present to a lesser degree on the westerly coastlines (Bandeira et al. 2014b). On the upper beach, at the edge of the dune thicket, species such as Canavalia rosea, Cissus quadrangularis and Cynanchum gerrardii have been recorded (Hyde 2021). This vegetation then transitions to coastal scrub further inland, featuring Diospyros rotundifolia and Euclea natalensis. Within this IPA, this vegetation type is most defined around Ponta Torres, the most south-easterly point of the island, however, dune scrub is usually continuous with the adjacent coastal thicket (Bandeira et al. 2014b). Coastal thicket features the Near Threatened species Encephalartos ferox alongside species such as Brexia madagascariensis and Brachylaena discolor. This latter species also occurs within the dune forest further inland, where species such as Afzelia quanzensis, Eugenia capensis, Mimusops caffra and Sideroxylon inerme dominate (Bandeira et al. 2014b).
According to Paula et al. (2014), Maputo Bay is home to six mangrove species Avicennia marina, Rhizophora mucronata, Ceriops tagal, Bruguiera gymnorhiza, Xylocarpus granatum, and Lumnitzera racemosa. The eastern coastlines of Maputo Bay at KaNyaka (and Machangulo Peninsula to the south) hold extensive mangrove communities. A dwarf form of the mangrove species A. marina is the dominant species covering the outer edges of the island particularly the less inundated areas. Whereas the muddy areas with rather less variable salinity have been colonised by Rhizophora mucronata. There are also thicket formations, within the mangrove mosaic, which are dominated by C. tagal and B. gymnorhiza.
The mangroves are bordered inland by saltmarshes (Lötter et al., in prep.), which include sedges such as Cyperus papyrus, grasses such as Phragmites australis alongside other herbs including Hibiscus cannabinus and Persicaria decipiens and the succulent Sesuvium portulacastrum (Hyde et al. 2021).
Much of the rest of KaNyaka consists of open woodland and savanna (Bandeira et al. 2014b). Trees such as Acacia, likely A. karroo as there are numerous mentions of this species in habitat descriptions within this site (Groenendijk #1353, #1532, #1942), Afzelia quanzensis, A. adianthifolia, A. versicolor and Dichrostachys cinerea dominate this habitat around Maputo Bay, with grasses in the understory including Hiperthelia dissoluta and Cymbopogon sp. (Bandeira et al. 2014b). Mogg (1967) noted the conspicuous absence of Brachystegia species from the woodland on KaNyaka, with only one individual of B. tamaridoides present on Portuguese Island, suggesting that this absence of this genus, ubiquitous throughout much of Mozambique, was due to the relatively recent emergence of this island. The woodland at this site is the most impacted by conversion of land to agriculture and includes scrub areas that have been previously used for subsistence agriculture but were later abandoned (Campbell et al. 1988). In such areas, dominant species include low shrubs and herbs such as Helichrysum kraussii, Cassytha filiformis, Digitaria eriantha, Tephrosia purpurea, Dicerocaryum zanguebarium, and Imperata cylindrica (Campbell et al. 1988).
In the intertidal zone surrounding KaNyaka are extensive seagrass meadows, covering around half of these areas of the coast of this island (Bandeira et al. 2014a). In total there are nine seagrass species documented from these waters, largely occurring within Maputo Bay (see Bandeira et al. 2014a for full species zonation patterns). Most significant is the large area of Zostera capensis (VU) in the bay and sand bank between KaNyaka and the Machangulo Peninsula.
Conservation issues
KaNyaka is an area of significant conservation importance which has long been recognised with the first form of formal conservation of this area established in 1965. The importance of this IPA is goes beyond the national level, falling within the Maputaland CoE (van Wyk 1996), which is part of a global biodiversity hotspot Maputaland-Pondoland-Albany (CEPF 2010). In recognition of the island’s tropical biodiversity, a Marine Biological Research Station was established in 1951 (Muacanhia 2004).
However, despite being a sanctuary for biodiversity, this island has been experiencing ongoing pressure for many years. Over half a century ago, Mogg (1967) found that forests together with freshwater swamps were under threat due to human encroachment, mainly for subsistence farming. In addition, the island has a dynamic environment that exhibits varying rates of erosion and sedimentation. For example, ongoing erosion resulting from strong wind is progressively depleting the southern east point known as Ponta Torres. The western section at Barreira Vermelha is experiencing degradation due to both tidal and freshwater erosion which causes landslides particularly in the rainy season (Muacanhia 2004).
After the establishment of the research base at KaNyaka Island in 1951 and, given the continuing increase of local population within this island, Portuguese authorities during the colonial era established the Forest and Marine Reserves in 1965 to protect the ecosystems and biological richness of the island (Muacanhia 2004). However, an increase in the local population which, together with extreme poverty experienced by local communities, poor soils and limited land due to the establishment of forest reserves, have increased pressure on land and resources in the IPA (Muacanhia 2004). To address these issues, in 2009 Inhaca Reserves were incorporated in the newly created POMPR (Ponta do Ouro Marine Partial Reserve) and subsequent to this, in late 2021, approval was given for the merger of POMPR with Maputo Special Reserve to form a new national park, Maputo National Park. This change in status should afford greater protection to the dune and intertidal habitats in the IPA.
Ponta do Ouro Partial Marine Reserve starts at the border with South Africa and extends north for 86 km following the coast into Maputo Bay, including KaNyaka Island, covering the base of the dunes to three nautical miles offshore throughout (Lucrezi et al. 2016). In 2019, the Maputo Environmental Protection Area (APA) was designated, covering the area from the POPMR northwards through the Maputo Special Reserve to as far north as KaNyaka Island. An APA is a conservation category under what is regarded as an “Area of Conservation for Sustainable Development” in accordance with the new Conservation Law 5/2017. This conservation category covers a broad landscape within which there may be included some existing protected areas and communities. Therefore, it allows an integrated management of landscapes (including managing existing protected areas or establishing new ones within it) to facilitate implementation of conservation, industrial development, among other development initiatives. An application to UNESCO has been prepared proposing that the area from Ponta do Ouro to KaNyaka should be recognised as a World Heritage Site (Matimele & Timberlake 2020). The full application covers various habitats (terrestrial and marine) and would naturally link with iSimangaliso World Heritage Site across the border in South Africa.
Ecosystem services
As with other islands in Mozambique, communities on KaNyaka Island rely on artisanal fishing and tourism as their primary livelihood (Book 2012). Because the island is part of the Ponta do Ouro Partial Marine Reserve (POPMR), there are “multiple use zones” where communities’ fishing ground are located.
In ecological terms, mangroves provide habitat for a wide range of fauna species including coastal and offshore fish and shellfish which have the mangroves as their main sanctuary for breeding, spawning, and hatching. The mangrove communities, along with primary dune vegetation, provide a buffer between the marine and terrestrial areas as well as protecting shorelines from destructive winds and waves. Mangrove and seagrass communities also contribute to climate regulation due to their role in carbon sequestration. For dugong that still exist around KaNyaka, their diet primarily consists of seagrass species found in the meadows around the island. The mangrove forests enhance water quality through filtering pollutants and terrestrial sediments. In addition, the mangroves and dune vegetation on the island serve as the main barrier protecting coastal erosion. The presence of forest patches contributes to carbon sequestration providing clean air.
Fruits of the trees Strychnos spinosa, Syzygium cordatum, Sclerocarya birrea subsp. caffra are consumed locally, while naturalised species such as guava (Psidium guajava) are also grown for their fruits or can be used as shade trees or for wind protection. As mentioned in the “Botanical Significance” section, Sclerocarya birrea subsp. caffra (Marrula) alongside with Vangueria infausta, Strychnos spinosa, Garcinia livingstonei, are of cultural significance for local communities. Marrula has a history of being deliberately encouraged by local people, it is used to make a beverage associated with celebrations, as a shade tree and its soft timber used to make utensils (Mogg 1967).
Site assessor(s)
Hermenegildo Matimele, Instituto de Investigação Agrária de Moçambique
Sophie Richards, Royal Botanic Gardens, Kew
Salomão Bandeira, Universidade Eduardo Mondlane
Iain Darbyshire, Royal Botanic Gardens, Kew
IPA criterion A species
| Species | Qualifying sub-criterion | ≥ 1% of global population | ≥ 5% of national population | 1 of 5 best sites nationally | Entire global population | Socio-economically important | Abundance at site |
|---|---|---|---|---|---|---|---|
| Ecbolium hastatum Vollesen | A(i) |  |
 |
 |
 |
 |
Unknown |
| Solanum litoraneum A.E.Gonç. | A(i) |  |
 |
 |
 |
 |
Occasional |
| Tephrosia forbesii Baker subsp. forbesii | A(i) |  |
 |
 |
 |
 |
Unknown |
| Tephrosia forbesii Baker subsp. inhacensis Brummitt | A(i) |  |
 |
 |
 |
 |
Occasional |
| Psychotria amboniana K.Schum. subsp. mosambicensis (E.M.A.Petit) Verdc. | A(i) |  |
 |
 |
 |
 |
Common |
| Adenopodia schlechteri (Harms) Brenan | A(i) |  |
 |
 |
 |
 |
Unknown |
| Dioscorea sylvatica Eckl. | A(i) |  |
 |
 |
 |
 |
Unknown |
| Zostera capensis Setch. | A(i) |  |
 |
 |
 |
 |
Abundant |
Ecbolium hastatum Vollesen





Solanum litoraneum A.E.Gonç.





Tephrosia forbesii Baker subsp. forbesii





Tephrosia forbesii Baker subsp. inhacensis Brummitt





Psychotria amboniana K.Schum. subsp. mosambicensis (E.M.A.Petit) Verdc.





Adenopodia schlechteri (Harms) Brenan





Dioscorea sylvatica Eckl.





Zostera capensis Setch.





General site habitats
| General site habitat | Percent coverage | Importance |
|---|---|---|
| Marine Intertidal - Mud Shoreline and Intertidal Mud Flats |  |
Major |
| Marine Coastal/Supratidal - Coastal Sand Dunes |  |
Major |
| Forest - Subtropical/Tropical Dry Forest |  |
Minor |
| Savanna - Moist Savanna |  |
Major |
| Wetlands (inland) - Seasonal/Intermittent Saline, Brackish or Alkaline Lakes and Flats |  |
Minor |
| Marine Intertidal - Sandy Shoreline and/or Beaches, Sand Bars, Spits, etc. |  |
Minor |
Marine Intertidal - Mud Shoreline and Intertidal Mud Flats

Marine Coastal/Supratidal - Coastal Sand Dunes

Forest - Subtropical/Tropical Dry Forest

Savanna - Moist Savanna

Wetlands (inland) - Seasonal/Intermittent Saline, Brackish or Alkaline Lakes and Flats

Marine Intertidal - Sandy Shoreline and/or Beaches, Sand Bars, Spits, etc.

Land use types
| Land use type | Percent coverage | Importance |
|---|---|---|
| Nature conservation |  |
Minor |
| Tourism / Recreation |  |
Minor |
| Agriculture (arable) |  |
Major |
Nature conservation

Tourism / Recreation

Agriculture (arable)

Threats
| Threat | Severity | Timing |
|---|---|---|
| Residential & commercial development - Housing & urban areas | Medium | Ongoing - trend unknown |
| Residential & commercial development - Commercial & industrial areas | Medium | Ongoing - trend unknown |
| Agriculture & aquaculture - Annual & perennial non-timber crops - Small-holder farming | High | Ongoing - increasing |
| Agriculture & aquaculture - Marine & freshwater aquaculture - Subsistence/artisinal aquaculture | Low | Ongoing - trend unknown |
| Biological resource use - Logging & wood harvesting | Medium | Ongoing - trend unknown |
Residential & commercial development - Housing & urban areas
Residential & commercial development - Commercial & industrial areas
Agriculture & aquaculture - Annual & perennial non-timber crops - Small-holder farming
Agriculture & aquaculture - Marine & freshwater aquaculture - Subsistence/artisinal aquaculture
Biological resource use - Logging & wood harvesting
Protected areas
| Protected area name | Protected area type | Relationship with IPA | Areal overlap |
|---|---|---|---|
| Ponta do Ouro Marine Partial Reserve | National Reserve | protected/conservation area overlaps with IPA | 70 |
Ponta do Ouro Marine Partial Reserve
Conservation designation
| Designation name | Protected area | Relationship with IPA | Areal overlap |
|---|---|---|---|
| Ponta do Ouro | Key Biodiversity Area | protected/conservation area overlaps with IPA | 70 |
Ponta do Ouro
Management type
| Management type | Description | Year started | Year finished |
|---|---|---|---|
| Protected Area management plan in place | In 2019, the Maputo Environmental Protection Area (Área de Protecção Ambiental – APA) was designated, covering the area from the Ponta do Ouro Partial Marine Reserve northwards through the Maputo Special Reserve to as far north as KaNyaka Island. An APA is a conservation category under what is regarded as an “Area of Conservation for Sustainable Development” in accordance with the new Conservation Law 5/2017. | 2019 |  |
Protected Area management plan in place

Bibliography
The endemic plants of Mozambique: diversity and conservation status
PhytoKeys, Vol 136, page(s) 45-96 Available online
Trees and Shrubs Mozambique
Zanthoxylum delagoense. The IUCN Red List of Threatened Species 2016: e.T38743A85955359
Historical Vegetation Map and Red List of Ecosystems Assessment for Mozambique – Version 1.0 – Final report
Epiphytic Seaweeds and Invertebrates Associated with South African Populations of the Rocky Shore Seagrass Thalassodendron leptocaule — a Hidden Wealth of Biodiversity.
African Journal of Marine Sciences, Vol 35, page(s) 523-531
Thalassodendron leptocaule. The IUCN Red List of Threatened Species 2020: e.T149255832A149275898
Distribution and Status of Zostera capensis in South African Estuaries - A Review
South African Journal of Botany, Vol 107, page(s) 63-73 Available online
Diversity and Distribution of Seagrasses around Inhaca Island, Southern Mozambique
South African Journal of Botany, Vol 68, page(s) 191-198
Seagrass Meadows in Maputo Bay
The Maputo Bay Ecosystem, Vol Chapter 8 (pub. WIOMSA), page(s) 147-169
The Terrestrial Environment Adjacent to Maputo Bay
The Maputo Bay Ecosystem, Vol Chapter 12 (pub. WIOMSA), page(s) 239-254
Secondary Dune Succession on Inhaca Island, Mozambique
Vegatatio, Vol 78, page(s) 3-11
Thalassodendron leptocaule – a New Species of Seagrass from Rocky Habitats
The Maputo Bay Ecosystem, Vol Chapter 8.2 (pub. WIOMSA), page(s) 175-179
Late Quaternary Sedimentary History of Inhaca Island, Mozambique
Transactions of the Geological Society of South Africa, Vol 80, page(s) 183-191
Flora of Zimbabwe: Utilities: Location search results: Inhaca Island
Tephrosia forbesii subsp. inhacensis. The IUCN Red List of Threatened Species 2019: e.T120979692A120980463
Solanum litoraneum. The IUCN Red List of Threatened Species 2021
The Fauna and Flora of Sand Flats at Inhaca Island, Moçambique
Journal of Animal Ecology, Vol 31, page(s) 93-128 Available online
Comments on the flora of Inhaca Island, Moçambique
Ecological Assessment and Biogeography of Coastal Vegetation and Flora in Southern Mozambique
Department of Botany, Faculty of Science; NELSON MANDELA UNIVERSITY
Maputaland World Heritage Application: Terrestrial Plants and Vegetation. Unpublished
Environmental changes on Inhaca Island, Mozambique: development versus degradation
The Impact of Sea-level Change, Past, Present, Future: Boletim Geológica, Vol 43, page(s) 28-33
Mangroves of Maputo Bay
The Maputo Bay Ecosystem, Vol Chapter 7 (pub. WIOMSA), page(s) 109-126
Ponta Do Ouro Partial Marine Reserve
Zostera capensis. The IUCN Red List of Threatened Species 2010: e.T173370A7001305
Designing a Transfrontier Conservation Landscape for the Maputaland Centre of Endemism Using Biodiversity, Economic and Threat Data
Biological Conservation, Vol 141, page(s) 2127-2138
Solid Waste Management at Inhaca Island
Biodiversity of the Maputaland Centre
The Biodiversity of African Plants (pub. Kluwer Academic Publishers), page(s) 198-207
Dioscorea sylvatica Eckl. National Assessment: Red List of South African Plants version 2020.1
Geomorphology and evolution of Maputo Bay
The Maputo Bay Ecosystem (pub. WIOMSA)
Recommended citation
Hermenegildo Matimele, Sophie Richards, Salomão Bandeira, Iain Darbyshire (2024) Tropical Important Plant Areas Explorer: Inhaca Island (Mozambique). https://tipas.kew.org/site/inhaca-island/ (Accessed on 27/07/2024)


